A spectrophotometer is an instrument that passes a selected wavelength of electromagnetic radiation through a solution and measures the amount of that electromagnetic radiation that the solution absorbs (does not allow to pass) or transmits (does allow to pass). Refer for a moment to the electromagnetic spectrum shown on the linked page, (from Life, The Science of Biology, by Purves et al., 5th ed., 1998). One type of spectrophotometer uses ultraviolet (UV) wavelengths; those are the wavelengths that cause sunburn, for example. Another type uses infrared (IR) wavelengths, which are most familiar as heat. You may have an opportunity to use one of these in another course. The third type of spectrophotometer, which we will use in this course, uses visible wavelengths, which we think of as the different colors of light, ranging from violet to red. Since these are the familiar colors, this type of spectrophotometer is called a "colorimeter." The particular model we will use is the Spectronic 20 colorimeter.
Since you will use the Spectronic 20 colorimeter in three quite different types of lab activities this semester, you must understand how it works and how to use it properly. Colorimetry is a procedure in which a colorimeter is used to analyze the composition of solutions, in one of two ways, as described below. We could also call this visible spectrophotometry, since we're using visible wavelengths. UV spectrophotometry and IR spectrophotometry would use ultraviolet or infrared wavelengths, respectively; of course, those would require different instruments, since the Spectronic 20 is designed to produce and detect only visible wavelengths.
The phenomenon of electromagnetic radiation being absorbed is already familiar to you in many ways. For example, exposure of one's skin to sunlight long enough produces a "sunburn." That's the result of molecules within skin cells absorbing the UV radiation in sunlight. The purpose of a sunblock rubbed on the skin is to provide other molecules to absorb that UV radiation before it reaches the skin. The point? Molecules do absorb electromagnetic radiation, and that absorption can be detectedů. a sunburn hurts! In another familiar example, the water molecules within foods absorb the microwave radiation (wavelengths at the long end of the infrared) in a microwave oven. The easily detectable result of that is that the food gets hot!
Suppose we add a drop of green food coloring to a cup of water and a drop of yellow food coloring to another cup of water. Each of these food colorings (dyes) is a different type of organic molecule. Imagine putting these two solutions into separate test tubes and holding them against the window light. You see them as different colors because the dye molecules in solution absorb (i.e. filter out) different wavelengths of light and transmit (i.e. allow to pass) different wavelengths to your eye. The green solution appears green because that type of dye molecule absorbs primarily wavelengths other than green. Similarly, the yellow solution appears yellow because it is the wavelengths in the yellow part of the spectrum that the molecules in solution do not absorb; so it is primarily the yellow wavelengths that reach your eye after passing through the solution. Thus, the quality of the light (quality means type, kind) that is absorbed depends upon the type of molecule dissolved in the solvent. Different types of molecules, such as green dye versus yellow dye, will absorb different types (wavelengths) of light.
The quantity of light that is absorbed depends on the concentration of the dissolved solute that is absorbing the light. More light of a given wavelength will be absorbed if you increase the concentration of the solute. To use the food dye example again, two drops of green dye per cup of water will produce a solution that appears slightly deeper green than a solution made with only one drop of green dye. Both solutions are absorbing the same non-green wavelengths, but the quantity of non-green light absorbed is greater in the more concentrated solution. This distinction between the quality and the quantity of light absorbed (or transmitted) by a solution is an important one in biology: qualitative properties or phenomena (what kind or type) versus quantitative properties or phenomena (how much/many).
In some types of lab tests the investigator wants to know which wavelengths of the spectrum are absorbed by solutes in a solution. This may help to identify a solute, since different types of molecules absorb different wavelengths. Refer to the absorption spectra (spectra is plural of spectrum) of photosynthetic pigments shown on the linked page (from Life, The Science of Biology, by Purves et al., 5th ed., 1998). Note that each pigment has a different profile of wavelengths that it absorbs more or less strongly. That profile is a trace of "peaks" and "valleys" and some peaks may have "shoulders" on their slopes. Understand that an absorption spectrum is a graph of light absorption (dependent variable) as a function of wavelength (independent variable). Again, it may be possible to identify a type of molecule on the basis of its characteristic profile (pattern) of wavelengths absorbed. You will produce such an absorption spectrum in today's lab, to see how easily this can be done.
In other types of lab tests the investigator wants to determine the concentration of a specific solute in a solution. In this sort of application of colorimetry the absorption spectrum of the solute of interest has already been determined. So, on the colorimeter we select a wavelength that the solute is known to absorb strongly. Referring to the graph of absorption spectra again, for the pigment called phycoerythrin 565 nm would be the best wavelength. We might say that's the wavelength location of the highest "peak" in the absorption spectrum of phycoerythrin. To use that green dye example again now, a series of test tubes containing different concentrations of the dye would all appear green but different depths of green. That means the solutions would all absorb the same wavelengths but different amounts of the light. Your eye could see relative differences in the depth of green among the tubes, but the colorimeter would quantify those differences for you, that is, assign numerical values to how much of the light entering the solution was absorbed and how much was transmitted. The relative amount of light of a given wavelength that is absorbed by a solution is called absorbance (A), whereas the fraction of light entering the solution that passes through unimpeded is called percent transmittance (%T). You will use this quantitative aspect of colorimetry next week in lab when you perform the biuret procedure to measure the protein concentrations of solutions.
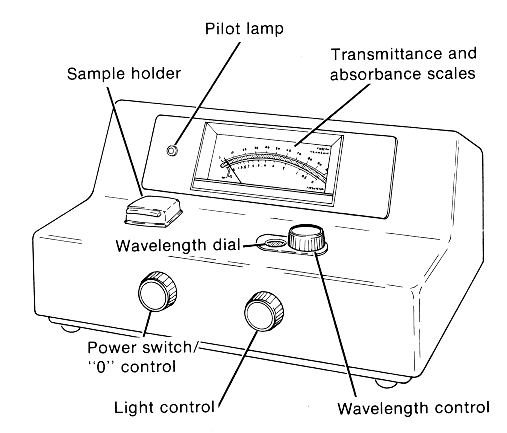
Observation of the visible spectrum inside the Spectronic 20 colorimeter
1. Turn the power switch clockwise to turn on the instrument; you'll hear/feel the on-click. The pilot lamp should glow, and you may hear the gentle sound of the cooling fan inside. If the pilot lamp doesn't glow on one of these instruments, don't be concerned. For the moment you may ignore the other uses of the two knobs on the front of the instrument.
2. Refer to the illustration of the instrument below. Each solution that you will test is placed into a small test tube, called a colorimeter tube, which is inserted into the sample holder. The beam of light, of whatever wavelength is selected, passes through the tube in the holder from right to left.
3. In your test tube rack is a colorimeter tube containing a rectangular strip of white paper that is a bit shorter than the length of the tube and just wide enough to fit snugly into the colorimeter tube. Insert this tube into the holder (pushing it all the way down); rotate the tube so that the faint light beam entering the tube from the right will strike the paper's flat surface.
4. Cup your hand around the top of the open sample holder and look closely down into the tube to see the faint light reflected off the paper. You will need to have your eye close to your cupped hand (near the top of the tube) because the light is faint. If the room light is too bright the instructor will turn off the room lights for a moment.
5. As you turn the wavelength control knob, you will see the light reflected by the paper change from violet/blue (about 400 nm) to green to yellow to orange and finally to red (about 700 nm). The lamp inside the instrument produces white light (a mixture of all wavelengths), but the wavelength control knob screens all but the one you select. In normal operation (i.e. without the paper in the colorimeter tube) the light that you see reflected would enter the tube containing a test solution and some portion of it would pass out the other (left) side of the tube to strike a detector, which would convert that light into an electrical signal that registers on the transmittance and absorbance scales.
6. Remove the tube from the sample chamber and replace it in the test tube rack.
7. In normal operation the lid of the sample holder is always closed after inserting the sample tube, before recording measurements, to shut out stray light.

Standardization of the colorimeter for determining the absorption spectrum of riboflavin, the vitamin
As light passes through a solution in a colorimeter tube, some small fraction of that light may be absorbed by the glass of the tube and by the solvent in which the molecule of interest is dissolved. Further, as you will see in your next lab exercise (the biuret test for measuring protein in solutions), the solution in the tube may contain other substances that are necessary to produce the color that you measure. Since many types of molecules are not colored, they must be reacted with something that will produce a color before you can use the colorimeter to measure them. Anything that the light passes through may absorb some of the light and influence measurements. Since the point is to measure absorption of one, particular type of molecule in a solution, it is necessary to correct for (to "subtract") light absorption by everything else (the glass of the test tube, the solvent, any color-producing reagents present). To do that you standardize the instrument before doing your measurements.
Riboflavin is a water soluble vitamin. The yellow solution in one of the tubes in your rack contains riboflavin dissolved in water, 0.017 mg/mL; nothing else is present. In order to get the most accurate possible data for determining the absorption spectrum of riboflavin, you must standardize the colorimeter to correct for light absorption by the glass and the solvent (water in this case). Therefore, you have another tube containing only water, that is, everything except the molecule of interest, riboflavin; this second tube is called the blank. You use the blank to standardize the instrument. In effect you will be "telling the instrument to ignore" light absorption by everything except the molecule of interest, riboflavin.
The steps in standardization are few and simple, but they must be done correctly and carefully to obtain reliable data.
1. If the instrument were not already turned on, you would use the power switch to turn on the instrument and let it warm up for about 15 minutes.
2. Use the wavelength control knob to select the desired wavelength; the wavelength values shown in the wavelength dial display window are graduated in nanometers (nm). NOTE WELL that in next week's work you will use only one wavelength; today, though, you will measure light absorption at many wavelengths in order to determine the absorption spectrum of riboflavin. So, we'll begin with 340 nm for the wavelength. Set that now.
3. With the sample holder empty and its lid closed, use the "0" control knob (same as the power control knob) to set the indicator needle to zero percent on the transmittance scale, 0 %T. Without a tube in the chamber, no light enters the chamber (it's pitch black in there), so no light would strike the detector. In effect, you're telling the instrument that if there were a tube with a solution in the chamber, this is what you'd see if absolutely none of the light entering the tube passed through to the other side, i.e. 0 % of the light got through.
4. Insert the blank tube into the holder and close the lid. The needle will sweep upward on the %T scale. Wherever the needle stops, use the light control knob now to move the needle to 100 %T on the scale. In effect, you're telling the instrument that with a sample in the chamber, but no riboflavin present, this is what you'd see if all of the light (100 % of it) entering the tube passed through to the other side. Since the glass and the solvent will also be present with your riboflavin sample, this step tells the instrument to ignore light absorption by those factors. Now, if you see any %T values less than 100%, that must be due to light absorption by the riboflavin; with riboflavin present, less than 100% of the light entering the tube will pass through.
5. Remove the blank from the sample holder and close the lid. The needle should fall back to zero %T.
6. The instrument is now standardized for 340 nm wavelength. If you
were going to do measurements on many samples at this single wavelength,
you would not touch the light control knob or the "0" control
knob hereafter.
Determining riboflavin's absorption spectrum
An absorption spectrum plots absorbance (A), not % transmittance, on the y-axis, as a function of wavelength on the x-axis. Absorbance is the other scale on your colorimeter. Note that absorbance and %T scales run in opposite directions; than means that they are reciprocally related; as one rises, the other falls. But note also the spacings between divisions on the scales; %T has a linear scale whereas absorbance has a logarithmic scale (common log, i.e. base 10 log). Specifically, A = log (1 / T), where T is the decimal equivalent of %T. Example: if %T = 50%, then T = 0.5 and (1 / T) = 2.0, and A = 0.301. Make sure you understand this algebraic relationship.
To collect data for riboflavin's absorption spectrum now, you must record absorbance values at many wavelengths, not just one, across the visible spectrum. It will be sufficient to take measurements at 5 nm increments. Each time the wavelength is changed, however, the 100 %T setting must be redone because the glass and solvent may not absorb light to the same degree at different wavelengths. But this extra step takes little time. So, proceed as follows.
1. With the colorimeter already standardized for 340 nm wavelength, insert your riboflavin tube, close the chamber lid, and record the absorbance reading. Don't change any knob settings yet. Then remove the riboflavin tube and close the lid. The needle will fall back to 0 %T, which is A = "infinity" (the maximum absorbance possible). You set the 0%T only once.
2. Reset the wavelength to 5 nm higher than the last setting. Insert the blank tube, close the lid, and note that the needle doesn't go all the way back to 100 %T. That's because the tube's glass and the water absorb different amounts of light at different wavelengths. So, reset the needle to 100 %T with the light control knob now. The machine is now standardized for the new wavelength.
3. Remove the blank tube, and see that the needle falls back to 0 %T (= maximum absorbance). Insert the riboflavin tube into the sample holder, close the lid, and record absorbance at the new wavelength. Don't adjust any knobs with the riboflavin sample in the chamber. Then remove the riboflavin tube and close the lid.
4. Now repeat steps #2 and #3: increase the wavelength in 5 nm steps, reset the 100 %T with the blank each time, and record a new absorbance value for the riboflavin solution. Your last wavelength setting will be 530 nm. This process goes quickly, but it must be done carefully.
5. Return both the blank tube and the riboflavin tube to the test tube rack. Never leave a sample in the instrument. Turn off the instrument by turning the power switch counter clockwise; you'll hear/feel the click.
6. Plot your data on graph paper that has both axes with linear scales: absorbance on the y-axis and wavelength on the x-axis. What is riboflavin's absorption maximum wavelength? How many "peaks" does the absorption spectrum have? Check your results with your lab instructor.